Matthias Beckmann 1,2, Matthew F.S. Rushworth 1,3, Heidi Johansen-Berg 1
- Centre for Functional MRI of the Brain, University of Oxford, Oxford, UK
- Medizinische Fakultät "Carl Gustav Carus", Technische Universität Dresden, Dresden, Germany
- Department of Experimental Psychology, University of Oxford, Oxford, UK
From HBM 2007 abstract site: http://www.meetingassistant3.com/ohbm2007
Introduction
Human cingulate cortex is involved in many different functions including conflict monitoring, decision making, movement and pain. This functional variability is reflected in anatomical specialization: the cingulate is composed of multiple, cytoarchitectonically distinct, sub-regions (1), each with distinct patterns of anatomical connectivity revealed by tracer studies in non human animals (2, 3, 4). Given the importance of the cingulate in human diseases (5, 6, 7), the ability to identify and investigate these sub-regions in vivo in the human brain would be beneficial. Here, we tested the possibility of defining anterior, mid, and posterior human cingulate sub-regions with automated probabilistic tractography.
Methods
Diffusion- and T1-weighted images were acquired in 11 healthy volunteers (1.5 T Siemens Sonata, 60 diffusion directions, b=1000 smm-2, 2x2x2mm voxels). Image analysis used FSL (www.fmrib.ox.ac.uk/fsl). Cingulate cortex masks served as seeds for multi fibre probabilistic tractography. (8) 'Blind' connectivity-based parcellation (9), with k means clustering (10), was used to define subregions with similar anatomical connections. Then, we used tractography to calculate the probability of connection between every cingulate voxel and pre-defined regions of interest (ROIs).
Results and Discussion
Connectivity-based parcellation of cingulate cortex identified three sub-regions with distinct patterns of connectivity, oriented from anterior to posterior and consistent across subjects (Fig. 1).
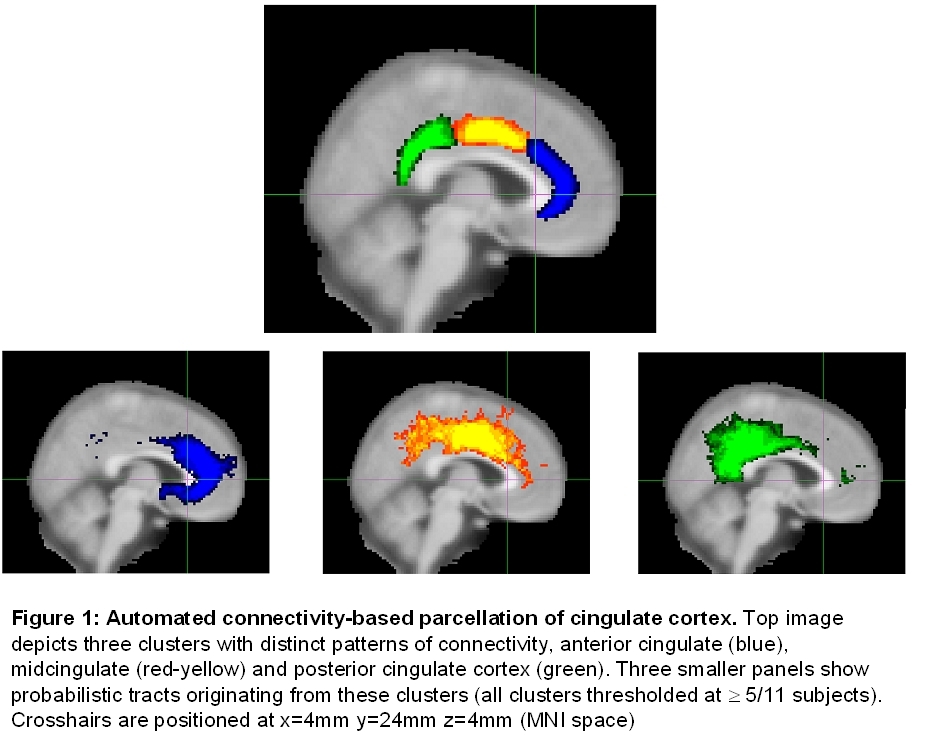
Classification of cingulate voxels according to their probability of connections with target ROIs resulted in clearly distinct clusters of voxels with specific spatial distribution (Fig. 2).
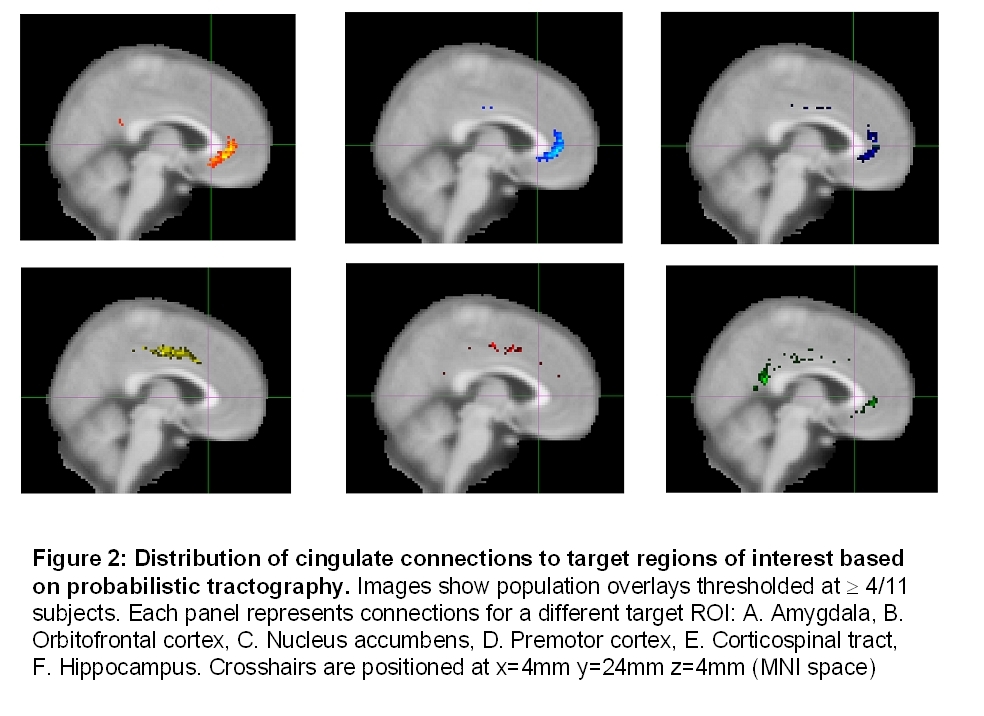
The anterior and subgenual cingulate cortex has strong connections with brain structures carrying reward and reinforcement information such as the nucleus accumbens, amygdala, and orbitofrontal cortex, consistent with our previous study of the subgenual region specifically (11). The midcingulate proved to be part of the motor network with connections to the premotor cortex and the corticospinal tract. In other primates the hippocampus connects prominently to two cingulate regions, the most posterior and the most anterior. A similar pattern was observed in the current study. Mapping these clusters onto the initial connectivity-based parcellation (Fig. 3) suggested that these connections may have been improtant determinants of the automated parcellation carried out in the first stage of the analysis.
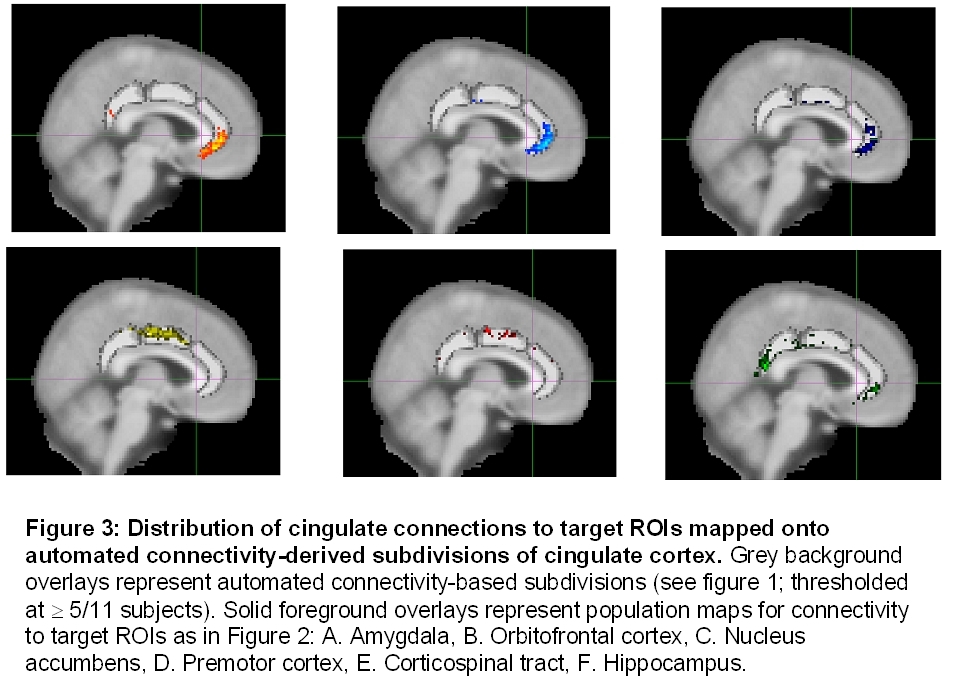
In conclusion, our results demonstrate the possibility of identifying sub-regions of the human cingulate cortex, in vivo, by virtue of their different patterns of anatomical connectivity.
References: 1. Vogt, J Comp Neurol, 1995; 2. Shibata, Eur J Neurosci, 2003; 3. An, J Comp Neurol, 1998; 4. Freedman, J Comp Neurol, 2000; 5. Mayberg, Neuron, 2003; 6. Kubicki, J Psychiatr Res, 2005; 7. Vogt, Exp Neurol, 1998; 8. Behrens, NeuroImage, 2007; 9. Johansen-Berg, Proc Natl Acad Sci USA, 2004; 10. Klein, NeuroImage, 2006; 11. Johansen-Berg, Proc OHBM, 2006
Category = Neuroanatomy Subcategory = DTI studies, application